Most of us seldom question whether or not the items we buy regularly are risk-free. Unfortunately, many consumer goods are incorrectly designed, manufactured, or marketed. These blunders might result in serious harm or […]
In collaboration with Johnson & Johnson, the DePuy Orthopedics developed a device in 2005 which sought to alleviate issues brought on by hip degradation. The DePuy ASR XL Acetabular System consists of a ball and joint mechanism made of material such as chromium and other materials that have been shown to withstand constant movement and rotation over the years.
The DePuy hip replacement system was approved by the FDA shortly after it was introduced to the country. However, it was not required to undergo rigorous testing because the federal body claimed that it closely resembled another device that was already on the market.
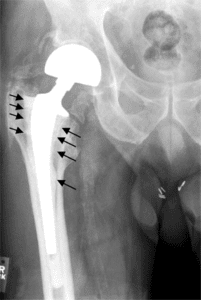
The DePuy device was found to have several defects shortly after its launch, given its rate of failure. It was shown that the device could disintegrate over time and even move from one part of the leg to a different section (metallosis). This can lead to issues such as tissue necrosis and a host of nervous system disorders.
There were reports of people developing cystic masses, non-cancerous tumors, inflammation, constant pain radiating from the groin and onto the back and other areas of the body, as well as fluid collection at different parts of the body.
Additionally, many of those who received the DePuy's ATTUNE® knee implant are reporting premature device failure (often due to loosening of the tibial base plate), with some reports of failure occurring within the first two months of surgery.
By 2008, the FDA had already started receiving reports of the medical device failing, a year before the company manufacturing it decided to stop selling it. In 2010, DePuy released a statement which said that the device has a higher than usual failure rate garnered from information collected in Australia by other patients who had had it implanted into them.
Despite the fact that DePuy announced a settlement in late 2013 to compensate victims of this defective product, we believe that the amount agreed on via a class action suit brought forward by thousands of people isn’t enough.
Please contact the product liability lawyers at Rasansky Law Firm if you’ve have a DePuy knee or hip implant and we’ll see how best to get you the maximum amount of compensation to help you undergo corrective surgery as well as compensate you for your pain, suffering and other damages.
Call us today at (214) 651-6100 for you free initial consultation. Thanks, and we look forward to hearing from you.
The attorneys at Rasansky Law Firm are happy to speak to you about your potential case free of charge. If we can help with your claim, we'll do so for no out-of-pocket cost to you. Call us 24/7 at (214) 651-6100.
Note: The information that was utilized in this post was gathered from the use of secondary sources. This information used has not been confirmed or independently verified. If you locate any information that is not correct, please contact our firm as soon as possible so that we can make the appropriate corrections. If you find any information that is false, we will remove or correct the post immediately after it is brought to our attention.
Disclaimer: As a valued member of the Dallas community, Rasansky Law Firm’s goal is to improve the safety of all residents in the great state of Texas. These posts should not be viewed as a solicitation for business and the information included herein should not be taken as medical or legal advice. The photos used in this post are not representative of the actual crash scene.
Over 30+ Years Of Personal Injury Experience
Top-Rated and Award-Winning Personal Injury Lawyers
Attorneys Available to Discuss Your Case Now
No Fee Unless You Win
Free Confidential Consultation.
Fill out the form below to receive a free and confidential initial consultation with an experienced personal injury lawyer.